Press release: PIC/S Meetings in Chicago (USA)
From 24 to 28 September 2018, the United States Food and Drug Administration (US FDA) hosted the following events in Chicago, Illinois (USA): PIC/S Committee meeting, PIC/S Executive Bureau meeting and PIC/S Annual Seminar.
HEADLINES
|
- NEW PIC/S GUIDANCE ON CLASSIFICATION OF GMP DEFICIENCIES ADOPTED
- PIC/S 2018 SEMINAR ON "MANAGEMENT OF RISK THROUGH THE PRODUCT LIFE-CYCLE" HOSTED BY US FDA ATTRACTS MORE THAN 200 INSPECTORS FROM 46 COUNTRIES
- NEW AMENDEMENT OF PIC SCHEME INITIATED
- BULGARIA / BDA APPLIES FOR ACCESSION AND JORDAN / JFDA APPLIES FOR PRE-ACCESSION
- BILATERAL MEETINGS WITH CHINA / NMPA AND INDIA / CDSCO
- IMPLEMENTATION OF PIC/S GUIDANCE ON GMP INSPECTION RELIANCE
- PIC/S PRIORITIES AND WORKPLAN FOR 2019
- NEW STAGE REACHED IN DEVELOPMENT OF PIC/S INSPECTORATES' ACADEMY
- LATEST PIC/S DEVELOPMENTS IN THE FIELD OF GM(D)P HARMONISATION
|
PIC/S COMMITTEE MEETING
|
PIC/S Chairman, Mr Boon Meow Hoe (Singapore / HSA)
|
The PIC/S Committee met on 24-25 September 2018, under the chairmanship of Mr Boon Meow Hoe (Singapore's Health Sciences Authority / HSA). The meeting was attended by 38 out of 52 PIC/S Participating Authorities (PA) as well as by a number of Applicants, Pre- Applicants, and Associated Partner Organisations.
|
PIC/S Committee meeting in Empire room at historical venue of Palmer House Hotel,
Chicago (USA)
|
|
|
The Committee meeting was opened by the PIC/S Chairman and the US FDA Associate Commissioner for Regulatory Affairs, Ms Melinda Plaisier. On behalf of the U.S. Food and Drug Administration’s (FDA) Commissioner of Food and Drugs, Dr. Scott Gottlieb, Ms Plaisier delivered welcoming remarks in which she highlighted in particular the goals and challenges that public health inspectorates share:
"1. We must work together to harmonize drug inspection programs across all sites of manufacture, domestic and foreign, so that we can inspect regulated products in the same way. Common training will be one way to work toward addressing that goal.
|

Associate Commissioner for Regulatory Affairs, Ms Melinda Plaisier (US FDA)
|
2. As science, technology, manufacturing, and production continue to evolve at a record pace, we also need to encourage adoption of emerging technologies to strengthen our inspectional approaches and assessments so that we can stay a step ahead of the industries we regulate. This is another challenge where common training can help us keep abreast of industry advancements.
3. The globalization of the manufacturing industry and supply chain is simply huge and no one inspectorate can inspect it all. Wherever possible, inspectorates need to share information and discuss our respective regulatory decisions in ways where we can learn from each other.
This means enhancing our workforce and culture, improving our core operations, leveraging and expanding our partnerships like PIC/S, and improving our IT infrastructures that support the vital work we do to protect public health.
PIC/S has made tremendous progress building collaborations among pharma inspectors. The impact that you have had on harmonizing the inspectors who enforce the regulations of GMPs and GDPs cannot be overstated."
The Associate Commissioner’s full speech is available for downloading at the following link: https://www.fda.gov/NewsEvents/Speeches/ucm622205.htm
NEW PIC/S GUIDANCE ON
CLASSIFICATION OF GMP DEFICIENCIES
The PIC/S Committee adopted a new Guidance on Classification of GMP Deficiencies (PI 040-1), developed by the PIC/S Working Group on Classification of Deficiencies, chaired by Australia / TGA.
The adoption of this guidance, which is scheduled to enter into force on 1 January 2019, is a major achievement by PIC/S, in line with PIC/S' mission which is to lead the international development, implementation and maintenance of harmonised GMP standards and quality systems of inspectorates in the field of medicinal products.
This guidance provides a tool to support the risk based classification of GMP deficiencies from inspections and to ensure greater consistency amongst inspectorates. It will enable industry to be informed of the principles used to classify GMP deficiencies and also provide examples of the classification of different types of deficiencies. This approach is not binding as the classification takes also into account the context of the finding and the quality history of the site. It does not remove the responsibility of the company in assessing the impact of the finding on the products already on the market and/or on their quality system.
The purpose and scope of this guidance is the harmonisation of the classification of GMP deficiencies to facilitate harmonised reporting of GMP deficiencies from inspections across inspectorates. Harmonisation will help ensure that there is a consistent view across inspectorates of what constitutes a “critical” deficiency and what constitutes a “major” deficiency. Risk management principles will be applied to the categorization of these deficiencies dependent on the type of product manufactured or process. The reference in the relevant code of Good Manufacturing Practice (GMP) or local legislation should be established for each deficiency to ensure that a reported deficiency has a regulatory basis and is accurately applied.
The guidance also intends to provide actions to be taken by inspectorates in response to the reporting of critical and major deficiencies; and enhance communication, information sharing and scientific exchange to promote increased consistency and predictability in regulatory assessments and decisions and the rapid exchange of safety and quality information regarding manufacturers.
Work on this guidance started back in 2012 following discussions on the classification of GMP deficiencies at the 2011 PIC/S Seminar in South Africa which led to PIC/S publishing a comparison of the top GMP deficiencies cited by the PIC/S Participating Authorities.
Since then a number of drafts of the guidance have been developed and discussed within the PIC/S Committee and PIC/S Sub-Committee on GM(D)P Harmonisation as well as with PIC/S inspectors, in particular during the 2016 and 2017 PIC/S Seminars. It is planned in the future that its scope will be further extended also to Good Distribution Practice (GDP) deficiencies.
This new guidance (PI 040-1) will be published on the page “Publications” prior to its entry into force.
2018 PIC/S ANNUAL SEMINAR ON
MANAGEMENT OF RISK THROUGH THE PRODUCT LIFE-CYCLE
 |
 |
PIC/S 2018 Seminar audience, Chicago (USA)
|
From left to right: US FDA Assistant Commissioner for Medical Products and Tobacco Operations, Ms Ellen F. Morrison; PIC/S Deputy Chairperson, Ms Anne Hayes (Ireland / HPRA); PIC/S Chairman, Mr Boon Meow Hoe (Singapore / HSA); US FDA Associate Commissioner for Regulatory Affairs, Ms Melinda K. Plaisier; PIC/S Chair of Sub-Committee on Strategic Development and US FDA Senior Advisor Medical Products, Ms Susan Laska (US FDA)
|
The 2018 PIC/S Annual Seminar was organised by the United States Food and Drug Administration in Chicago, Illinois (USA) on 26-28 September 2018.
The topic of the Seminar was “Management of Risk through the Product Life-Cycle”. The Seminar was opened by the US FDA Associate Commissioner for Regulatory Affairs, Ms Melinda Plaisier and the PIC/S Chairman, Mr Boon Meow Hoe.
The Seminar, which was the first organised in the USA since US FDA joined PIC/S in 2011, was attended by more than 200 inspectors from 46 countries. All continents were represented. This was the first time that India / CDSCO, Jordan / JFDA and Sri Lanka / NMRA attended a PIC/S Seminar.
Risk management is critical to ensuring product quality, safety, and efficacy. The Seminar explored the best practices impacting risk assessments and shared tools and techniques generated from experienced inspectors and assessors to enhance inspections. It was an ideal opportunity for both novice and experienced inspectors to refine their inspection skills through knowledge sharing and discussion.

For more information on the Seminar, see below.
NEW STAGE OF DEVELOPMENT FOR
PIC/S INSPECTORATES' ACADEMY
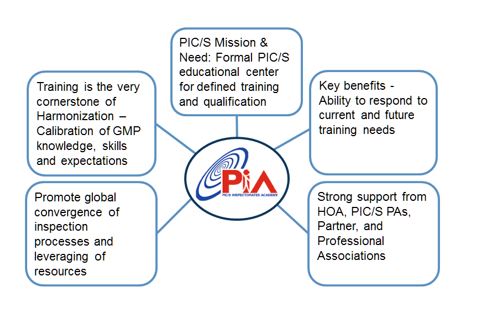
The PIC/S Inspectorates' Academy (PIA) is a Global Capacity Building and Training Initiative developed by PIC/S Participating Authorities aiming at delivering inspection excellence through harmonized training in the field of Good Manufacturing Practices (GMP) to ensure that high quality standards for medicinal products are met worldwide in the interest of public health.
PIA aims to provide training to improve inspection expertise in the manufacturing of medicines and of their distribution for regulators by regulators. It has been developed on the basis of PIC/S recognised GMP training experience and expertise since 1971. As a PIC/S initiative it is supported by PIC/S 52 Participating Authorities from all continents which regroup approx. 2,000 inspectors worldwide. It currently offers more than 500 training materials and 250 recorded training videos. Webinars, on-line learning tools and modules, a forum and library of relevant GMP references are in development.
Efforts to harmonise, improve and strengthen regulatory capabilities through training are of paramount importance, in particular the possibility to provide a pragmatic approach to ‘calibrate” GMP inspectors and to uphold consistency in the interpretation of GMP, the classification of GMP deficiencies, inspection methodology, inspection skills and inspectors’ qualification. Training also allows inspectors to keep abreast of the most recent best practices and standards as well as improve these. PIC/S training often results in the development or revision of existing GMP guidance.
5 Key Features of PIA
The Academy is an ambitious project whose development will be spanning several years in 3 stages of development. In 2016, stage 1 of the Academy was launched successfully with its website and the incorporation of all existing PIC/S training.
Revised Development Stages of PIA

Stages 2 & 3 are in development and will encompass a fully integrated learning management system extending the current training resources, on the basis of a harmonised training curriculum. This will include basic, intermediate and advanced levels, consisting in a fine balance between E-learning modules and Webinars designed to needs and face-to-face training. Delivery and monitoring are to be optimised and the training curriculum steps to result in recognised certification.
The PIC/S Committee discussed and endorsed in principle the next steps in the development of Stage 2 of PIA, subject to available funding and resources. In particular, it was agreed to re-prioritise some elements between Stage 2 and 3, in particular the development of a “training curriculum” which will be composed of three segments starting with the basic training and New Inspector Training Course. The Committee agreed on the need to update the identification of training needs and priorities for PIC/S, taking into account also opportunities for co-operation e.g. with the EU/EEA Heads of Medicines Agencies (HMA).
LATEST PIC/S DEVELOPMENTS IN THE FIELD
OF GM(D)P HARMONISATION
Following the public joint PIC/S – EMA – WHO stakeholder consultation on the revision of Annex 1 (sterile manufacturing) of the PIC/S, EU and WHO GMP Guides, which ended on 20 March 2018, more than 6,300 comments have been received and are currently being assessed by the Working Group on Annex 1, chaired by UK / MHRA. The PIC/S Sub-Committee on GM(D)P Harmonisation (SCH) Chair, Paul Gustafson (Canada / RORB), congratulated the Working Group on its outstanding efforts in advancing this revision and updated the Committee on the current status.
The revision of Annex 2 (manufacture of biological medicinal substances and products for human use) of the PIC/S GMP Guide is progressing. The PIC/S Working Group on Revision of Annex 2, chaired by Australia / TGA, is focusing first on the development of a new Annex which is currently being proposed to be Annex 2A for Advanced Therapy Medicinal Products (ATMP) with Annex 2B being the modified version of the existing Annex 2 for biologics. This approach aims to harmonise, where possible, with the European Commission’s (EC) Guidelines on GMP specific to ATMP, while addressing the concerns of PIC/S Participating Authorities.
An update was given on the status of the work of the EMA drafting groups in which PIC/S is represented for the revision of the PIC/S - EU GMP Guide Chapter 1 (Pharmaceutical Quality System); Chapter 4 (Documentation) and Annex 11 (Computerised Systems); and new Annex 21 (Imports). An update was also provided on progress by the PIC/S Sub-Committee on GM(D)P in the revision of Annex 13 (Investigational Medicinal Products) and in the development of a PIC/S version of EU Annex 16 (Certification by a QP & Batch Release).
A 3-month focused stakeholders’ consultation on the draft PIC/S guidance on Good Practices for Data Management and Integrity in Regulated GMP/GDP Environments (PI 041-1 (Draft 3)), developed by the PIC/S Working Group on Data Integrity, co-led by Australia / TGA and UK / MHRA, will shortly be launched. This focused consultation will seek substantive comments from trade and professional associations on specific questions, which selected associations have agreed to compile (ECA Foundation, IFPMA, ISPE and PDA). In parallel to this stakeholder consultation, PIC/S Participating Authorities will be invited to apply (PI 041-1 (Draft 3)) on a trial basis for a new implementation trial period. A PIC/S Aide-Memoire on Inspection of Data Management and Integrity as well as system-specific guidances - which are to remain PIC/S-internal and are currently under consultation - have also been developed by this Working Group.
The Drafting Group, led by Switzerland / Swissmedic, in charge of the revision of the PIC/S GMP Guide for Blood Establishments (PE 005-3) and PIC/S Guide to Inspections of Source Plasma Establishment and Plasma Warehouses (PE 008-3), with the aim to merge and harmonise these with the EU Good Practices Guidelines for blood establishments, has developed a first draft which will be discussed by the PIC/S Expert Circle on Human Blood, Tissues, Cells and ATMPs at its meeting in Warwaw (Poland) on 23-25 October 2018.
After review of comments received further to the consultation carried out with PIC/S PA in 2017, the draft PIC/S Aide-Memoire on Inspection of Manufacturers and Wholesale Distributors for Compliance with GDP and draft Q&A for the PIC/S GDP Guide, developed by the Expert Circle on GDP, chaired by UK / MHRA, will be discussed at the Expert Circle meeting on 16-18 October 2018 in Madrid (Spain).
The SCH is considering whether a limited revision of the PIC/S Guide to good Practices for the preparation of medicinal products in Healthcare Establishments (PE 010- 4) may be sufficient in order to include, as endorsed by the Committee at its last meeting, the EMA / EU Parenteral Nutrition guidance as an appendix or if this guidance may need a more complete revision / updating.
A revision of the PIC/S Recommendations on Validation Master Plan; Installation and Operational Qualification; Non-Sterile Process Validation; and Cleaning Validation (PI 006-3) is still underway.
The Committee discussed new developments and initiatives taken by ICH (International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use) which may have an impact in the field of GM(D)P.
AMENDMENT OF PIC SCHEME INITIATED
The process for a future amendment of the PIC Scheme (PICS 1/95 (Rev 5)), which gives PIC/S' legal basis as a non-profit association under Swiss law and governs its organisation and functioning, has been been initiated.
The last revision of the PIC Scheme dates back to 2012. The purpose of this new revision is:
- To clarify the definition of Member (“Participating Authority”) and Non-Member wishing to apply for membership (“Competent Authority”);
- To describe PIC/S’ legal status and explain what PIC/S is, why it has established; and
- To streamline the language.
A draft revision was submitted to the Committee for a first reading. The revision process, which involves several steps, is likely to take one year.
BULGARIA / BDA APPLIES FOR ACCESSION
JORDAN / JFDA APPLIES FOR PRE-ACCESSION
The Bulgarian Drug Agency (BDA) applied for PIC/S membership on 27 August 2018. A Rapporteur and Co-Rapporteur were appointed by the Committee. The PIC/S assessment process for BDA will be facilitated through the recognition of the EMA Joint Audit Programme (JAP) audit conducted in March 2017, in line with the Letter of agreement between PIC/S and EU/EEA Heads of Medicines Agencies (HMA).
The Jordan Food and Drug Administration (JFDA) applied for PIC/S pre-accession on 9 August 2018. A Rapporteur was appointed by written procedure subsequently to the Committee meeting.
PIC/S MEMBERSHIP APPLICATIONS
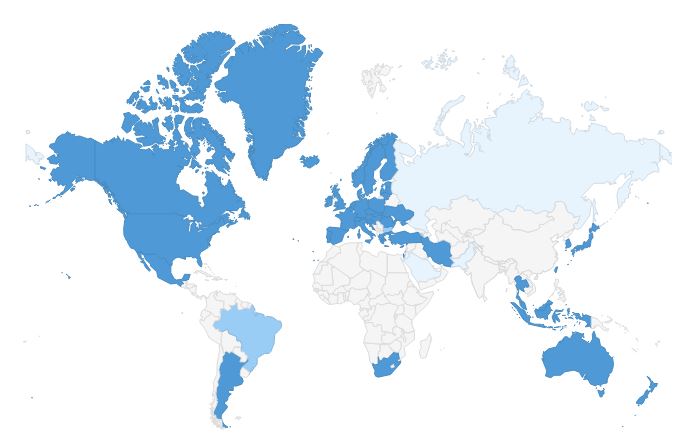
| Overview of PIC/S 52 Members as of 1 January 2018 (dark blue); 4 Applicants (medium blue) and 4 (Pre-) Applicants (pale blue) |
| |
Applicants (4) |
Pre-Applicants (4) |
| |
Armenia / SCDMTE
Bulgaria / BDA
Brazil / ANVISA
Italy (Vet) / DGSAF
|
Jordan / JFDA
Pakistan / DRAP
Russian Federation / Minpromtorg and
FSI SID&GP
Saudi Arabia / SFDA
|
|
- The Committee was updated on the status of the membership application of Armenia / SCDMTE, completed on 15 April 2018, which will be facilitated by the pre-accession gap-analysis;
- The Committee took note of the current absence of progress in the updating by Brazil / ANVISA of its membership application during the 1-year stop-clock granted by the Committee at its last meeting in lieu of the re-lodging of its membership application further to a re-organisation;
- The Rapporteur for the membership application of Italy (Vet) / DGSAF, which will be facilitated by a partial assessment, taking into account the audit performed within the EMA Joint Audit Programme, reported on the planning of an observed inspection in Q1 2019 in order to complete the assessment;
- The Committee was updated on the status of the assessment of the pre-accession membership application of Pakistan / DRAP for which a Rapporteur was appointed by written procedure on 25 June 2018;
- The Russian Federation / Minpromtorg Russia and FSI "SID&GP" provided an update on the status of the review and completion of their pre-accession documentation as well as gave a presentation on the Eurasian Economic Union and its GMP requirements;
- The Committee was updated on progress in the assessment by the Rapporteur of the pre-accession application of Saudi Arabia / SFDA;
- Following the gap-analysis for the pre-accession application of Kazakhstan / CCMPA closed on 17 November 2017, the Committeee reviewed a report by the Rapporteur on the CAPA provided on 13 February 2018 by CCMPA, which the Rapporteur has reviewed on a voluntary basis;
- The Committee welcomed the intent expressed by the Head of Agency of Philippines / PFDA to submit a new PIC/S membership application pending the possible positive outcome of the assessment by the ASEAN MRA Panel of Experts.
BILATERAL MEETINGS WITH
CHINA / NMPA AND INDIA / CDSCO
A bilateral meeting between the PIC/S Executive Bureau and a high-level Delegation from China's National Medical Products Administration / NMPA (formerly CFDA) took place on 27 Septembre 2018. This meeting allowed China / NMPA to update PIC/S on its recent re-organisation as well as enabled both PIC/S and NMPA to mutually discuss how to best address some issues raised by NMPA in connection with a possible future PIC/S application.
Further to recent interest expressed by the Drug Controller General of India in joining PIC/S, India's Central Drugs Standard Control Organisation / CDSCO was invited and attended the 2018 PIC/S Seminar. A bilateral meeting took place on 28 September 2018 with a high-level representative of CDSCO, which was the first occasion for such a meeting with the PIC/S Executive Bureau. The meeting allowed to discuss a number of questions relating to the possibility of a future application to PIC/S.
IMPLEMENTATION OF PIC/S GUIDANCE
ON GMP INSPECTION RELIANCE
At its last meeting, the Committee adopted the Guidance on GMP Inspection Reliance (PI 048-1) with entry into force on 1 June 2018. At its meeting in Chicago, PIC/S Members discussed how to monitor the (voluntary) implementation of the guidance by PIC/S PAs as well as non-PIC/S Members. Further to discussions in the PIC/S Sub-Committee on Strategic Development (SCSD) and the PIC/S Sub-Committee on Communication (SC COM) on how to promote the use of the tool and how metrics should start to be collected in 2019, the Committee adopted a new template.
This template will serve for the collection of statistics on foreign GMP inspections, which have been waived and replaced by a desktop assessment or by accepting the GMP certificate of another PIC/S Participating Authority (PA). The purpose of these statistics is to document the efforts made by PIC/S PAs to rely on existing inspection reports rather than duplicate (foreign) GMP inspections. With the new tool provided by this guidance, PIC/S PA have an option to make informed regulatory decisions whether to waive an on-site inspection (thereby avoiding duplication of efforts) or conducting a reduced scope inspection.
PIC/S PRIORITIES AND WORKPLAN FOR 2019
A Work Plan was adopted by the Committee for the year 2019 in line with the goals and priorities defined in the PIC/S Road Map for the period 2018-20. The Work Plan covers all areas of PIC/S activities: organisational, compliance, training and expert discussions, harmonisation of GM(D)P, strategic development and co-operation, communication and financing.
This Work Plan (PS/W 18/2018) will be published on the page “Publications” prior to the start of 2019.
OTHER NEWS
The PIC/S Committee:
- was updated on the outcome of discussions within the PIC/S Executive Bureau meeting on the morning of 24 September 2018, which preceded the Committee meeting;

(1) (2) (3) (4) (5) (6) (7) (8)
|
(1) PIC/S Sub-Committee on Strategic Development Chair, Susan Laska (US FDA)
(2) PIC/S Sub-Committee on Expert Circles Chair, Andreas Krassnigg (Austria / AGES)
(3) PIC/S Sub-Committee on GM(D)P Harmonisation Chair, Paul Gustafson (Canada / RORB)
(4) PIC/S Sub-Committee on Budget, Risk and Audit Chair, Ger Jan van Ringen (Netherlands / IGJ)
(5) PIC/S Deputy Secretary, Jeffrey Hodgson
(6) PIC/S Chairman, Mr Boon Meow Hoe (Singapore / HSA)
(7) PIC/S Sub-Committee on Training Chair, Jacques Morénas (France / ANSM)
(8) PIC/S Deputy Chairperson and Sub-Committee on Compliance Chair, Anne Hayes (Ireland / HPRA)
|
- adopted a mandate for the PIC/S Working Group on Veterinary Medicinal Products (VMP), led by France / ANSES and UK / VMD; and a mandate for the PIC/S Working Group on Inspector Travel Safety, led by UK / MHRA;
- adopted a new mandate for the Expert Circle on Good Distribution Practices (GDP), led by UK / MHRA;
- adopted new Guidelines on Donations developed in response to the need to plan for the possibility of third-party funding in support of some of PIC/S projects and was updated on the PIC/S Working Group in charge of third-party funding which will include active participation of the PIC/S Executive Bureau;
- discussed a proposal by the Chairman of the Working Group on the Drafting of Pre-Accession Guidelines, led by France / ANSM, to change the pre-accession process in order to allow Pre-Applicants to assess the gaps, based on their understanding of the PIC/S requirements as explained during the pre-accession process;
- discussed the topic of the 2019 PIC/S Seminar on "Quality Assurance of Sterile Medicinal Products - Annex 1" which will be hosted by Japan / MHLW and PMDA in Toyama city (Japan) on 13-15 November 2019. For the promotional video click here:

- accepted an invitation from Thailand / Thai FDA to host the 2020 PIC/S Seminar in Bangkok (Thailand) in November 2020 on a GMP topic yet to be defined;
- was updated on the planning of the PIC/S re-assessments of Switzerland / Swissmedic on 15 – 19 October 2018; of Ukraine / SMDC on 22 – 26 October 2018; and of Argentina / INAME on 5 – 9 November 2018;
- discussed the selection and composition of the PIC/S Re-Assessment Teams for the PIC/S re-assessments of Canada / RORB and South Africa / SAHPRA scheduled in 2019;
- decided to re-assess Indonesia / NADFC and New Zealand / Medsafe in 2020;
- was updated on progress accomplished by the PIC/S Working Group on Unique Facility Identifiers (UFI), led by US FDA and by the PIC/S – EU JAP Compliance Group Working Group on the guideline and interpretation of the Audit Checklist, led by Canada / RORB;
- was updated on the status of the Working Group on the Revision of the Accession Guidelines (and related documents) led by France / ANSM, which is soon to start work;
- was updated on the development of a draft mandate for the future PIC/S Working Group on Whistle-blowers / Confidential Informants, to be co-led by US FDA and UK / MHRA;
- was updated on the status of the PIC/S Working Group on the revision of the PIC/S guidance on Good Practices for Computerised Systems (PI 011-3);
- invited PIC/S PA to appoint nominees to join the PIC/S Working Group on Quality Defects Procedures in charge of transposing for PIC/S purposes the revised EMA procedures on (i) Managing Reports of Suspected Quality Defects in Medicinal Products; and (ii) Handling Rapid Alerts Arising from Quality Defects;
- was updated on current developments with regard to the implementation of the EU – US Mutual Recognition Agreement (MRA) and was updated on recent GMP-related developments by EDQM, EMA, UNICEF and WHO as PIC/S Associated Partner Organisations;
- was updated by the PIC/S – ASEAN Liaison Authority, Thailand / Thai FDA, on recent activities within ASEAN and on positive feedback from the ASEAN Pharmaceutical Product Working Group (PPWG) on an informal exchange of letters to allow for co-operation between PIC/S and the ASEAN PPWG in GMP matters.
RECENT AND UPCOMING TRAINING ACTIVITIES
|

|
- 11-13 September 2018: PIC/S Expert Circle on Quality Risk Management (QRM) and Advanced QRM Training, in Taipei (Chinese Taipei), hosted by Chinese Taipei / TFDA;
- 16-18 October 2018: PIC/S Expert Circle on Good Distribution Practices (GDP), in Madrid (Spain), hosted by Spain / AEMPS;
- 23-25 October 2018: PIC/S Expert Circle on Human Blood, Tissues, Cells and ATMPs, in Warsaw (Poland), hosted by Poland / CPI;
- 26-30 November 2018: 2018 Japan / PMDA - ATC GMP Inspection Seminar, with the support of PIC/S, in Tochigi (Japan), hosted by Japan / PMDA;
- 19-21 June 2019: PIC/S Expert Circle on Controlling Cross Contamination in Shared Facilities (CCCISF), in Taipei (Chinese Taipei), hosted by Chinese Taipei / TFDA;
- October 2019: PIC/S Expert Circle on Active Pharmaceutical Ingredients (API), in Madrid (Spain), hosted by Spain / AEMPS;
- 13-15 November 2019: Annual PIC/S Seminar on “Quality Assurance of Sterile Medicinal Products - Annex 1”, in Toyama city (Japan), hosted by Japan / MHLW & PMDA;
- dates to be confirmed in 2019: PIC/S New Inspector Training Course, Dublin (Ireland), hosted by Ireland / HPRA;
- dates to be confirmed in 2019: PIC/S Expert Circle on Human Blood, Tissues, Cells and ATMPs, in Indonesia (location to be confirmed), hosted by Indonesia / NADFC.
|
2018 PIC/S ANNUAL SEMINAR ON
MANAGEMENT OF RISK THROUGH THE PRODUCT LIFE CYCLE
|
Seminar Opening Address by
US FDA Associate Commissioner for Regulatory Affairs, Ms Melinda Plaisier
Ms Plaisier welcomed all Seminar participants to Chicago. It was an honour for US FDA to host this year's Annual Seminar. With just over 200 inspectors from 46 countries, she stated "such a strong turnout reflects the profound effects that globalisation is having on the regulatory landscape and the importance and value of us as regulators working together to leverage our knowledge, skills, and abilities."
|
She emphasised the role and responsibilities of inspectors for public health through their inspectional work, in particular as inspections are being used by other authorities to make decisions. The Seminar topic addressed the fact that regulators are continually assessing risk: from the risk that helps determine which sites to inspect to the risk of what critical aspects to inspect. These responsibilities, along with the growing complexity and emerging technologies of drug products, present regulatory challenges, but organisations such as PIC/S are instrumental in ensuring that inspectors from across the world are assessing GMPs in a harmonized way. She explained how US FDA has evolved in the face of the globalisation of drug manufacturing over the past years and mentioned that efforts, including co-operation and harmonisation, with other foreign regulators is critical to ensuring that the drug supply produced meets GMPs, stating that PIC/S is uniquely positioned to lead this harmonisation effort. She thanked inspectors for the work they carry out every day, looked forward to continued co-operation to ensure that PIC/S facilitates meaningful regulator-to-regulator collaborations and wished all participants a successful seminar.
The Associate Commissioner’s full speech is available for downloading at the following link: https://www.fda.gov/NewsEvents/Speeches/ucm622200.htm
The 2.5 day Seminar consisted of a mix of presentations and parallel workshops. The first day adressed Process and Production Plant Risks and started with a panel discussion outlining various Perspectives on Risk through the Product Life-Cycle from regulatory panellists from the US, Asia, Europe as well as from an invited Guest Industry speaker. A series of presentations then took place on Quality Oversight through the Upstream Supply Chain (for purchased raw materials and then for contracted services) and on Maintaining Process Control, followed by two parallel workshops on:
-
Risk Related to Raw Materials and Contract Services which allowed participants to discuss GMP requirements that relate to supplier qualification as well as exchange views on a number or real cases (Workshop leader: UK / MHRA);
-
Emerging Technologies, which included the review of a number of key aspects of Continuous Tableting Manufacturing (CTM), in particular Batch vs. CTM, CTM Controls and Operation Control Requirements (Workshop leader: Health Canada)
The second day focused on Risk to the Product with presentations on Quality Oversight through the Downstream Supply Chain: Finished Products and on Risk Based Inspections, followed by two parallel workshops on:
-
Risk in the Distribution Chain which enabled inspectors to review key risks in the distribution chain and to discuss preparations for such inspections as well as how to assess inventory control systems, track-and-trace and blockchain technologies (Workshop leaders: Singapore / HSA and New Zealand / Medsafe);
-
Risk Based Inspections which through a number of practical cases aimed at training inspectors to better prepare and carry out risk-based inspections (Workshop leader: Ireland / HPRA and US FDA).
The outcome and feedback resulting from these workshops were discussed on the last day, followed by a series of presentations dedicated to Risk to Patients and Personnel including Drug Shortage Risk and Inspector Safety. A panel discussion then provided Recap and Closing Perspectives on the content of the Seminar.
|




 PIC/S Committee meeting in Empire room at historical venue of Palmer House Hotel,
PIC/S Committee meeting in Empire room at historical venue of Palmer House Hotel,







